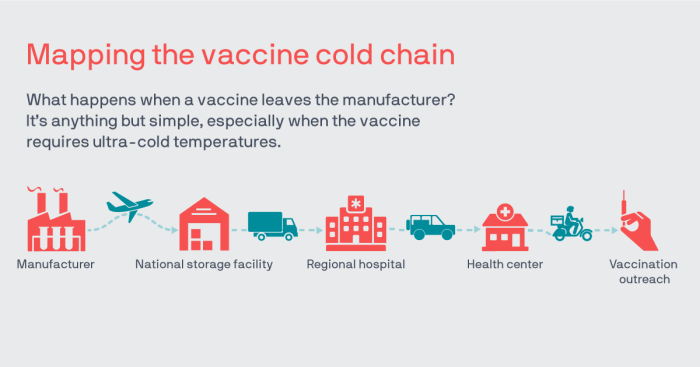
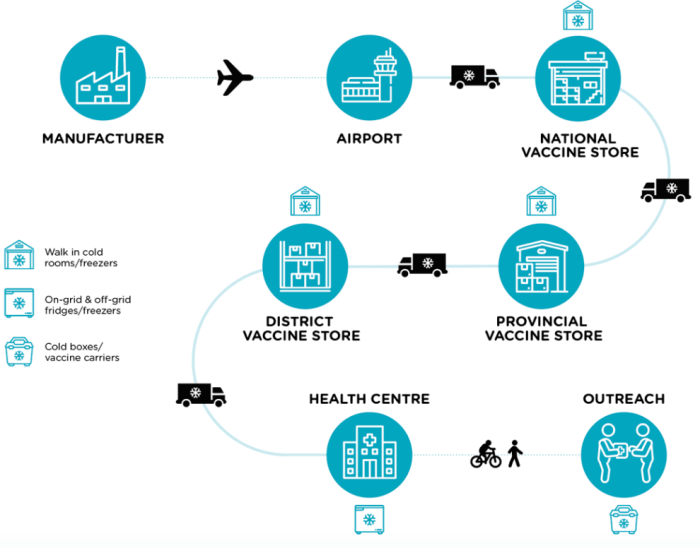
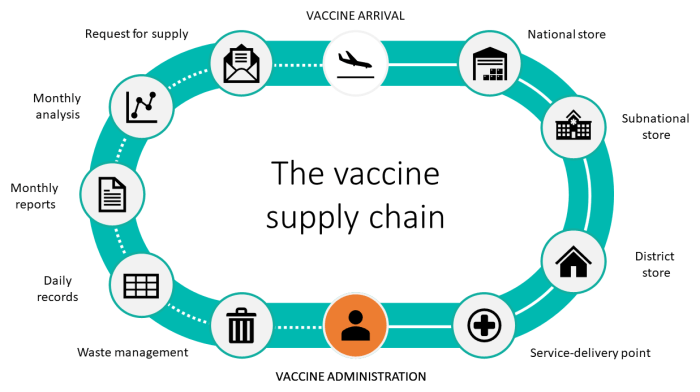
Seasonal influenza vaccine cold chain management for logistical personnel is a crucial aspect of public health efforts to prevent the spread of influenza. By maintaining the integrity of the cold chain, logistical personnel play a vital role in ensuring the potency and effectiveness of influenza vaccines.
This comprehensive guide delves into the key principles, best practices, and logistical considerations involved in seasonal influenza vaccine cold chain management. It provides essential knowledge for personnel responsible for vaccine storage, transportation, and administration, empowering them to effectively safeguard vaccine quality and protect public health.
Understanding Seasonal Influenza Vaccine Cold Chain Management: Seasonal Influenza Vaccine Cold Chain Management For Logistical Personnel
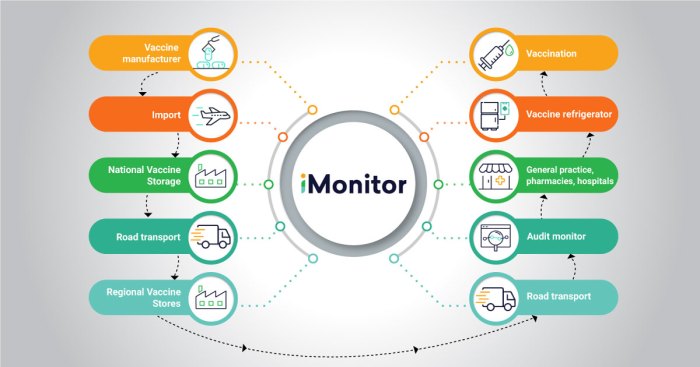
Maintaining the cold chain for seasonal influenza vaccines is crucial to ensure their potency and effectiveness. Improper cold chain management can lead to vaccine degradation, reduced efficacy, and potential safety concerns.
Challenges and risks associated with improper cold chain management include temperature fluctuations, inadequate storage facilities, and mishandling during transportation.
Key Principles and Best Practices
Effective seasonal influenza vaccine cold chain management involves adhering to key principles and best practices:
- Temperature Monitoring and Control:Vaccines must be stored and transported within a narrow temperature range (typically between 2°C and 8°C) using calibrated thermometers and data loggers.
- Storage and Handling Procedures:Vaccines should be stored in designated refrigerators or freezers with proper ventilation and away from potential contaminants. Proper handling techniques, such as using clean gloves and avoiding prolonged exposure to room temperature, are essential.
- Transportation Protocols:Vaccines should be transported in insulated containers with temperature-controlled packaging to maintain the cold chain during transit.
Logistical Considerations for Personnel
Personnel involved in seasonal influenza vaccine cold chain management have specific logistical considerations:
- Roles and Responsibilities:Different personnel, such as storage managers, transportation coordinators, and administrators, have distinct roles and responsibilities in maintaining the cold chain.
- Training and Certification:Personnel should receive comprehensive training and certification to ensure they understand cold chain principles and best practices.
Monitoring and Evaluation
Monitoring and evaluating the effectiveness of seasonal influenza vaccine cold chain management is crucial:
- Key Performance Indicators (KPIs):KPIs, such as temperature deviations, vaccine wastage, and administration rates, are used to assess cold chain integrity.
- Continuous Improvement and Corrective Actions:Regular monitoring allows for identification of areas for improvement and implementation of corrective actions to enhance cold chain management.
Case Studies and Best Practices
Successful seasonal influenza vaccine cold chain management practices include:
- Centralized Vaccine Storage:Establishing central storage facilities with rigorous temperature control and monitoring systems.
- Automated Temperature Monitoring:Utilizing automated temperature monitoring systems to track and record temperature data in real-time.
Future Trends and Innovations, Seasonal influenza vaccine cold chain management for logistical personnel
Emerging trends and innovations in seasonal influenza vaccine cold chain management include:
- Blockchain Technology:Exploring blockchain technology to enhance traceability and accountability in the cold chain.
- Smart Packaging:Developing smart packaging solutions with integrated temperature sensors and data loggers.
Popular Questions
What are the key principles of seasonal influenza vaccine cold chain management?
Key principles include maintaining proper storage temperatures, monitoring temperature fluctuations, and adhering to transportation protocols to preserve vaccine potency.
What are the roles and responsibilities of logistical personnel in cold chain management?
Logistical personnel are responsible for vaccine storage, transportation, and administration, ensuring adherence to cold chain protocols and vaccine integrity.
How can logistical personnel monitor the effectiveness of cold chain management?
Monitoring involves using temperature monitoring devices, assessing vaccine quality, and conducting regular audits to identify and address any deviations from established standards.