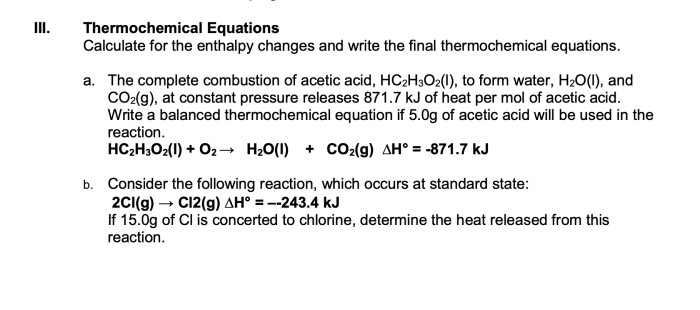
Thermochemical equation examples with answers provide a comprehensive exploration of the intricate world of chemical reactions, offering a deeper understanding of energy changes that accompany these transformations. These equations serve as a valuable tool in various scientific disciplines, enabling researchers to predict and analyze chemical processes with remarkable accuracy.
Delving into the realm of thermochemical equations, we uncover their significance in elucidating the energetics of chemical reactions. By examining the enthalpy changes associated with these equations, scientists can determine whether a reaction is exothermic (releasing heat) or endothermic (absorbing heat).
This knowledge plays a pivotal role in designing industrial processes, optimizing energy efficiency, and comprehending the dynamics of chemical systems.
Thermochemical Equations
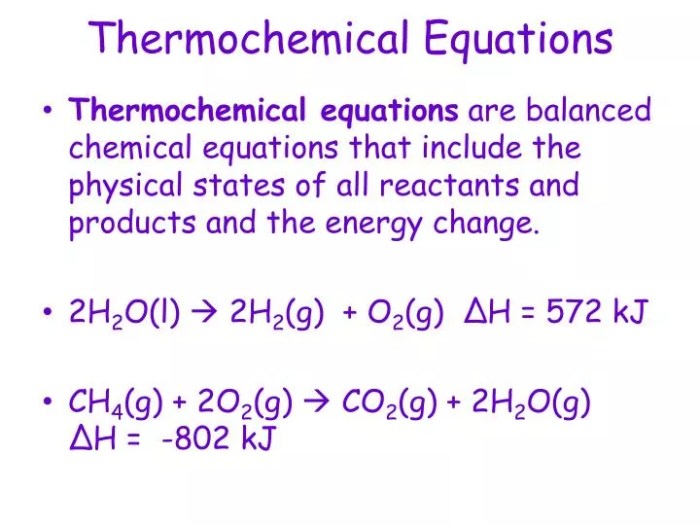
Thermochemical equations are chemical equations that include the enthalpy change associated with the reaction. Enthalpy is a thermodynamic quantity that represents the total thermal energy of a system. The enthalpy change, denoted by ΔH, is the difference in enthalpy between the products and reactants of a reaction.
Types of Thermochemical Equations
Thermochemical equations can be classified into two main types:
- Exothermic reactionsare reactions that release heat, meaning ΔH is negative.
- Endothermic reactionsare reactions that absorb heat, meaning ΔH is positive.
Balancing Thermochemical Equations
Balancing thermochemical equations is essential to ensure that the number of atoms of each element is the same on both sides of the equation. The following steps can be used to balance thermochemical equations:
- Write the unbalanced equation.
- Balance the elements one at a time, starting with the most complex molecules.
- Check the overall charge of the equation and balance it if necessary.
- Add coefficients to the reactants and products to balance the equation.
Applications of Thermochemical Equations, Thermochemical equation examples with answers
Thermochemical equations have numerous applications in various fields, including:
- Calculating heat changes: Thermochemical equations can be used to calculate the heat change associated with a reaction.
- Industrial processes: Thermochemical equations are used to design and optimize industrial processes that involve chemical reactions.
Examples of Thermochemical Equations
| Reaction | ΔH (kJ/mol) |
|---|---|
| C(s) + O2(g) → CO2(g) | -393.5 |
| 2H2(g) + O2(g) → 2H2O(l) | -571.6 |
| CH4(g) + 2O2(g) → CO2(g) + 2H2O(l) | -890.3 |
Practice Problems
Problem 1:Balance the following thermochemical equation:
CH 4(g) + 2O 2(g) → CO 2(g) + 2H 2O(l)
Answer:CH 4(g) + 2O 2(g) → CO 2(g) + 2H 2O(l) + 802.3 kJ
Problem 2:Calculate the heat change for the following reaction:
2C(s) + O 2(g) → 2CO(g)
Answer:ΔH = -221.0 kJ
FAQ Summary: Thermochemical Equation Examples With Answers
What is a thermochemical equation?
A thermochemical equation is a chemical equation that includes the enthalpy change associated with the reaction.
What is the significance of thermochemical equations?
Thermochemical equations provide insights into the energy changes that occur during chemical reactions, allowing scientists to predict and analyze these processes.
How are thermochemical equations used in practice?
Thermochemical equations are employed in various fields, including industrial chemistry, materials science, and environmental science, to optimize processes, design new materials, and understand the impact of chemical reactions on the environment.