Is nitric acid homogeneous or heterogeneous – As we delve into the intriguing realm of chemistry, the question of whether nitric acid is homogeneous or heterogeneous takes center stage. This discourse delves into the fundamental properties of nitric acid, exploring its molecular composition and behavior to unravel the mysteries surrounding its homogeneity.
Nitric acid, a potent and versatile chemical, exhibits a fascinating array of physical and chemical characteristics that shape its behavior in various contexts. Understanding the homogeneity of nitric acid is crucial for comprehending its reactivity, applications, and potential hazards.
1. Definition of Homogeneous and Heterogeneous Mixtures
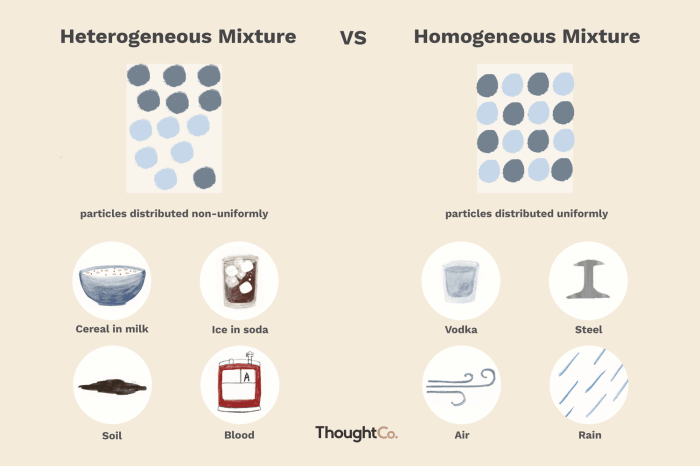
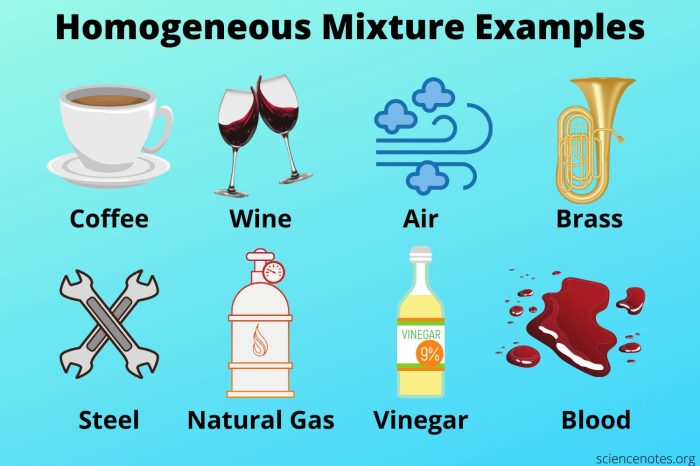
A homogeneous mixture is a mixture in which the components are evenly distributed throughout the mixture, resulting in a uniform composition and appearance. In contrast, a heterogeneous mixture is a mixture in which the components are not evenly distributed, resulting in a non-uniform composition and appearance.
Homogeneous mixtures are often referred to as solutions, while heterogeneous mixtures are often referred to as suspensions or colloids.
Characteristics of Homogeneous Mixtures
- Uniform composition and appearance throughout the mixture
- No visible particles or boundaries between the components
- Components are evenly distributed at the molecular level
Definition of Heterogeneous Mixtures
- Non-uniform composition and appearance throughout the mixture
- Visible particles or boundaries between the components
- Components are not evenly distributed at the molecular level
Examples of Homogeneous and Heterogeneous Mixtures
- Homogeneous mixtures:Salt water, air, milk, gasoline
- Heterogeneous mixtures:Sand in water, oil and water, salad dressing, granite
2. Properties of Nitric Acid: Is Nitric Acid Homogeneous Or Heterogeneous
Nitric acid is a highly corrosive, colorless, and fuming liquid with a pungent odor. It is one of the most important industrial chemicals and is used in a wide variety of applications.
Physical Properties of Nitric Acid
- Color: Colorless
- Density: 1.51 g/mL
- Boiling point: 83 °C
- Melting point: -42 °C
Chemical Properties of Nitric Acid, Is nitric acid homogeneous or heterogeneous
- Acidity: Nitric acid is a strong acid and reacts with bases to form salts.
- Oxidizing ability: Nitric acid is a strong oxidizing agent and can oxidize many metals and organic compounds.
- Corrosiveness: Nitric acid is highly corrosive and can cause severe burns on contact with skin.
3. Homogeneity of Nitric Acid
Nitric acid is a homogeneous mixture. This is because the components of nitric acid (hydrogen, nitrogen, and oxygen) are evenly distributed throughout the mixture at the molecular level. As a result, nitric acid has a uniform composition and appearance.
4. Applications of Nitric Acid

Nitric acid is used in a wide variety of applications, including:
- Fertilizer production:Nitric acid is used to produce ammonium nitrate, which is a common fertilizer.
- Explosives production:Nitric acid is used to produce explosives such as nitroglycerin and TNT.
- Metalworking:Nitric acid is used to etch and clean metals.
- Chemical synthesis:Nitric acid is used to produce a variety of chemicals, including dyes, plastics, and pharmaceuticals.
5. Safety Considerations for Nitric Acid
Nitric acid is a hazardous chemical and should be handled with care. The following safety precautions should be taken when handling nitric acid:
- Wear appropriate personal protective equipment, including gloves, goggles, and a lab coat.
- Work in a well-ventilated area.
- Avoid contact with skin and eyes.
- Store nitric acid in a cool, dry place.
- Dispose of nitric acid properly.
Essential Questionnaire
Is nitric acid a strong acid?
Yes, nitric acid is a strong acid that ionizes completely in water, releasing hydrogen ions (H+) and nitrate ions (NO3-).
What is the pH of nitric acid?
Nitric acid has a pH of approximately 1, indicating its highly acidic nature.
Is nitric acid corrosive?
Yes, nitric acid is highly corrosive and can cause severe burns on contact with skin or tissue.